
MEDICAL
Life sciences & healthcare enterprises are being significantly disrupted. It’s transforming healthcare, accelerating pharma’s shift to digital, and turning long-term planning upside down. We are uniquely positioned to partner with companies through our deep domain knowledge in the medical industry and our engineering expertise in Mechanical, Electronics, Software and Digital Technologies.
We enable our customers in the Medical Device industry to create the frontier for enhanced health care experience to improve the lives of millions of people around the world.
COMPLAINTS OF MEDICAL DEVICE

•ISO 13485 Standard - Quality Management System (QMS) for Medical industries.
•Complaints the US and EU medical device’s and instrument’s.
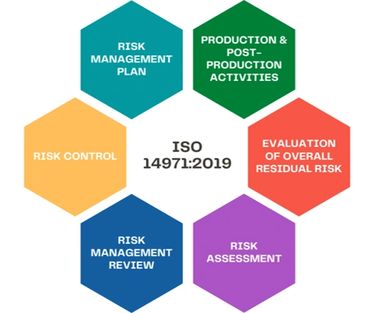
•ISO 14971 Standard - Provides a process for manufacturers to manage the risks associated with medical devices Risk Managements.
•Design Controls For medical device - Collection of documents that outline the design and development of a medical. device. According to the approved manufacturing plan and meets regulatory.
•Process Validation IQ/OQ/PQ - The protocol and report creation is a key part of process qualification that documents performance over time to ensure product quality.
•Ability to ensure the sterilization process and find the bio-combability of a material to interact with living tissue in a specific way, causing minimal adverse reactions such as inflammation or rejection.
•Performing the development support for the medical instruments to compliance with MDR updates.


Cookie Policy
We use cookies to enable essential services and functionality on our site and to collect data on how visitors interact with our site, products and services.
